Industrial Defoaming Agents
【Video of the verification of the effectiveness of solid antifoamers】
Defoamers Lineup
| Type | Product | Main Components | Feature |
|---|---|---|---|
| Silicone | Bisfoam ED-10 | Silicone Oil Emulsifier |
High foam-breaking and foam suppression properties |
| Bisfoam GC-302 | Silicone Oil Emulsifier |
High foam-breaking and foam suppression properties | |
| Polyether | Bisfoam CS | Higher alcohol derivatives | Generic products |
| Bisfoam ECC | Higher alcohol derivatives | Good effect even at low temperatures | |
| Bisfoam S-25 | Glycol-based modified silica |
Enhanced foam-breaking type | |
| Oil-based | Bisfoam K-301 | Mineral oil Metal soap |
high foam collapse property for use in coating applications |
| Bisfoam SS-2 | Mineral oil amide wax |
Strong defoaming, minimal surface defects ("hazing") | |
| Emulsion | Bisfoam TDI-1 | fatty alchols Emulsifier |
Economical product |
| Bisfoam TS-10 | Fatty acid derivative Emulsifier |
Highly water-dispersible | |
| Solid | Bisfoam BV | Silicone-based | No dosing equipment required (hang-in type) |
↓↓↓ Available now on Nissin Chemical Lab, the official online shop of Nissin Chemical Research Institute. ↓↓↓

Mechanism of action of defoamers
Antifoaming agents are intended to suppress foaming by adding a very small amount to the target foaming liquid. Foaming is a thin film of liquid that envelops air, and it is said that surface tension and foam viscosity are important factors in the generation of foam.
Therefore, it can be said that antifoam agents are those that partially unbalance the surface of the foam film and destabilize the foam.
(Foam collapse)
At the surface of a foam, surfactant molecules are arranged in an orderly manner with their hydrophobic tails oriented outward. In contrast, defoaming agents possess both hydrophilic and hydrophobic segments. When a defoamer is added, some of the surfactant molecules on the foam surface are replaced by defoamer molecules.
Normally, the thin film of a bubble maintains a certain thickness and exhibits high elasticity. However, the intrusion of the defoaming agent disrupts this structure, reducing the film’s elasticity and thinning it beyond its stability threshold, ultimately causing the film to collapse and the foam to break.
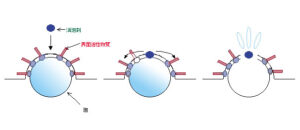
(Foam suppression)
By pre-dispersing defoaming agents into the foaming medium, the agents intrude into the surfactant layer aligned at the liquid surface, disrupting the interfacial structure. In addition, when bubbles reach the surface and attempt to form stabilized thin films, the defoaming agents hinder the film stability, effectively suppressing the formation of foam.
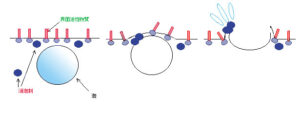
(Air release)
Defoaming components function as a coalescing agent within the foaming liquid, promoting the adhesion and merging of dispersed microbubbles. The resulting larger bubbles rise to the surface more quickly due to their higher buoyancy. Upon reaching the surface, these bubbles are eliminated through the two previously mentioned interactions—disruption of interfacial surfactant alignment and destabilization of the foam film.
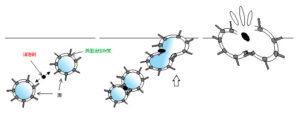
Types of defoamers
Based-Oil
These defoamers are formulated by dispersing active defoaming components into mineral oil or similar carriers. They are particularly effective for quickly breaking surface foam. They also exhibit excellent thermal resistance, making them suitable for high-temperature applications. One of their major advantages is low cost.
However, their drawbacks include limited longevity despite their rapid initial action. Due to their strong oil-based nature, they may cause oil separation, which can pose environmental concerns—particularly in wastewater applications where discharge into rivers or other natural water bodies is involved.
Surfactant-Type Defoamer
This type of defoamer is a kind of surfactant. Unlike conventional detergents, which are highly hydrophilic and tend to generate foam, this defoamer is highly hydrophobic and oil-based. When added to water, it becomes a milky dispersion above a certain temperature, earning it the name self-emulsifying defoamer. This temperature is referred to as the cloud point.
These defoamers have a high active ingredient content, typically between 90% and 100%, allowing for strong defoaming performance with only a small dosage. They also exhibit excellent stability and can be stored for long periods.
However, they are relatively expensive and require precise control of the dosage. Additionally, if the liquid temperature at the foaming site is below the cloud point, adding this defoamer may actually promote foaming instead of suppressing it.
Structure of Surfactant type defoamers
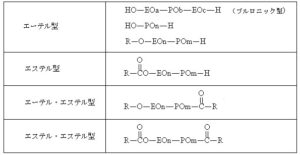
R: Alkyl group
EO: Ethylene oxide
PO: Propylene oxide
n, m, a, b, c: Arbitrary integers (degree of polymerization or substitution)
Among the surfactant-type defoamers mentioned above, one of the most representative types is composed of higher alcohols modified with ethylene oxide (EO) and propylene oxide (PO).
This corresponds to the ether-type structure shown in the table: R–O–(EO)_n–(PO)_m–H.
Adjusting the molar ratio of EO and PO allows for the design of defoamers optimized for specific foaming environments, enabling precise control over properties such as dispersibility, cloud point, and defoaming performance.
Surfactant-type defoamers exhibit molecular structures similar to those of common surfactants used in detergents and deinking agents—the primary distinction being the degree of EO and PO polymerization. Consequently, if not properly formulated or dosed, these defoamers may act as foaming agents themselves, leading to unintended foam formation.
Emulsion Type
This type of defoamer is an emulsion in which defoaming components (such as higher alcohols or esters) are dispersed in water. In order to activate its defoaming function, the active components are emulsified and dispersed in an aqueous medium, making it an O/W type (oil-in-water type) defoamer.
It offers excellent dispersibility in the target foaming system and is considered to have minimal impact on system properties such as particle size. It also demonstrates good deaeration performance.
However, one drawback is its sensitivity to storage conditions. Over time, thickening, solidification, or phase separation may occur, resulting in a decline in defoaming performance. To prevent such degradation, it is recommended to store the product indoors, away from direct sunlight.
As for the characteristics of each type of defoaming agent, it is generally said that oil types have excellent foam-breaking properties, activator types have excellent foam suppression properties, and emulsion types have excellent degassing properties. However, when trying to eliminate foam using each type alone, the characteristics alone may not produce satisfactory results as an antifoaming agent. In such cases, we recommend the use of different types of defoamers in combination, or the use of defoamers that also have other characteristics beforehand.
